DIY Cards

Do You Know Your Scientist?
Fact Card
Catherine, Emma, Mabel, Rachel POINTS
Born-Dec 15, 1852, Died- Aug 25, 1908

Quantum theory
Chemistry Mix n Match
The study of energy radiated from heated solids, and how energy behaved like particles in the form of tiny discrete energy packets or bundles called quanta. Waves could also behave like particles, with definite boundaries and bounced off each other when collided like particles do.

John Dalton
Chemistry Mix n Match
John Dalton was an English school teacher in the early 1800s. He was also the first to develop a model of the atom. He came up with the Atomic Theory as well.

Atomic Theory
Chemistry Mix n Match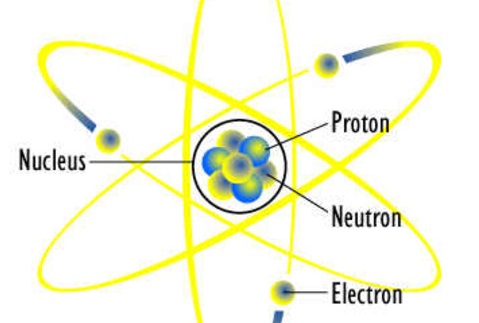
1) All matter is composed of extremely small particles called atoms that can’t be destroyed or created
2)Atom of any element are identical to one another
3)Atoms of different elements combine in specific ratios to form compounds
4)Chemical reactions,atoms are separated,rearranged,and recombined to form new compounds

Neutron
Chemistry Mix n Match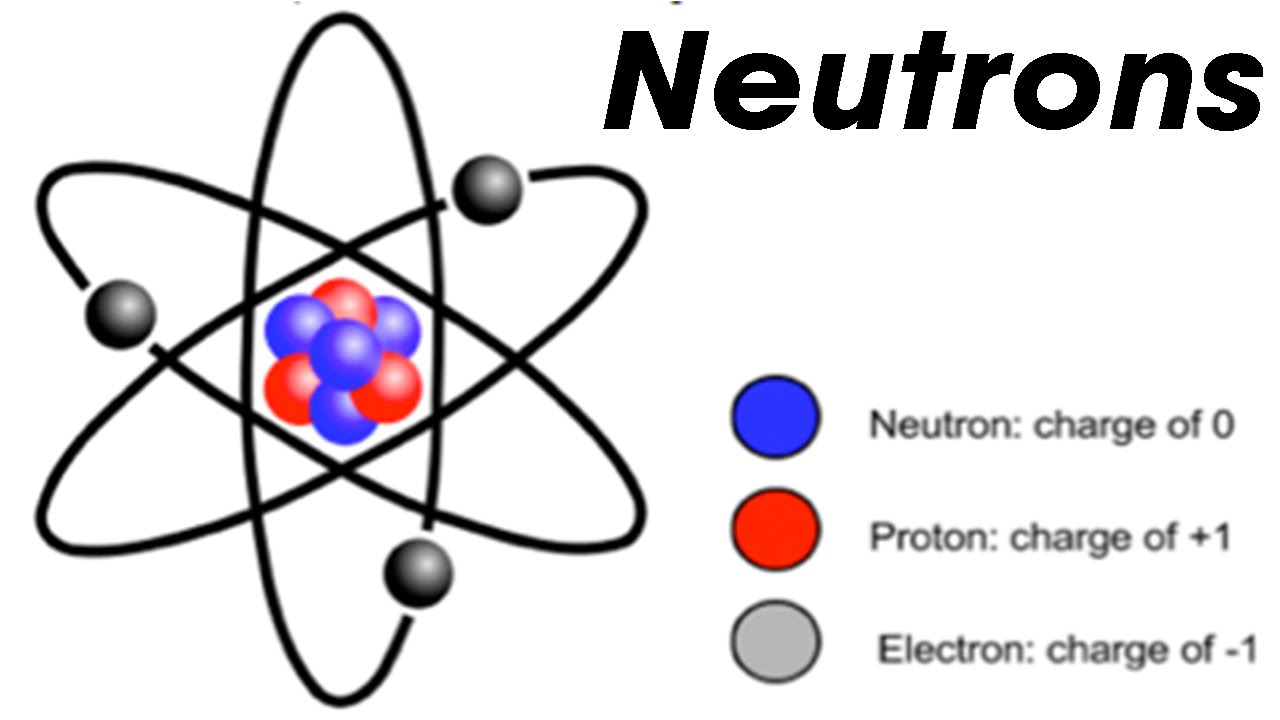
A particle discovered by the Curie-Joliot experiment and first mistaken-ed as gamma rays. It wasn’t effected by magnetic or electric fields and named neutrons because it didn’t posses a charge. It’s found in the nucleus.

Max Planck
Chemistry Mix n Match
A German Physicist in the 1900, proposed that energy could behave like particles in the form of tiny discrete energy packets or bundles he called quanta.