DIY Cards

Discovery/Theory card
The Periodic Law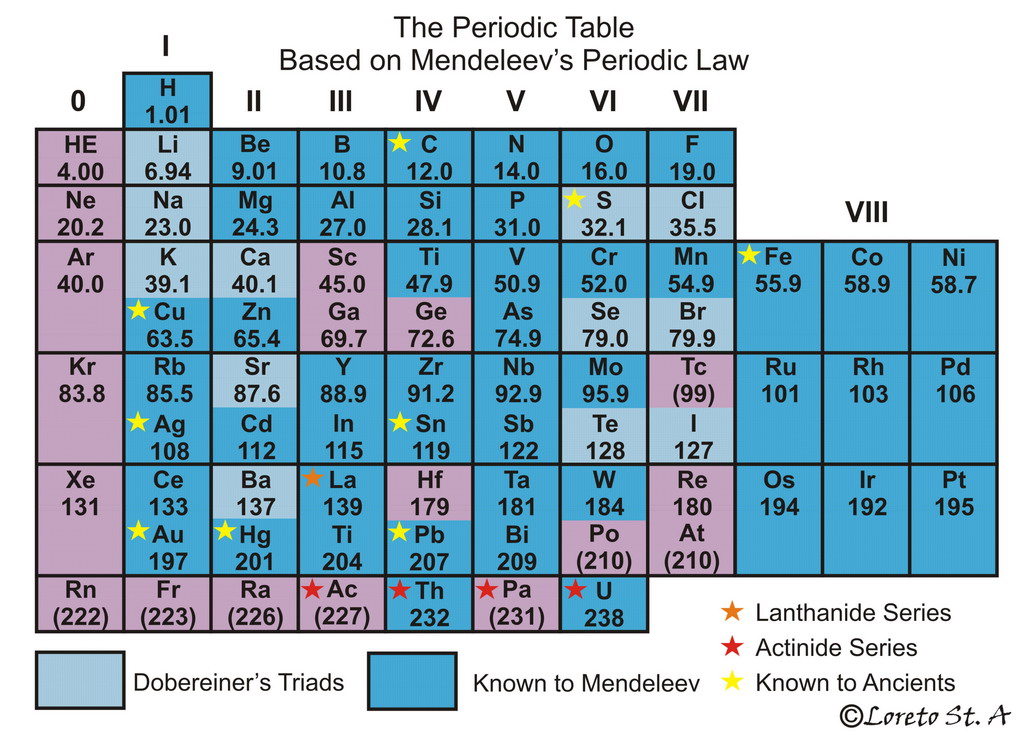
Parker POINTS
Repetition in chemical and physical properties of the elements is a fundamental pattern in nature / The pattern is periodicity and forms the basis of the periodic law / The properties of the elements recur periodically when the elements are arranged in increasing order by their atomic numbers

Dmitri Mendeleev
Chemist and Inventor 1834-1907
Parker POINTS
Home: Russia
Awards: Copley Medal, Demidov Prize, Element 101 – Mendelevium is named after him
Notable students: Konovalov, Gemilian
Quote: In a dream I saw a table where all the elements fell into place as required. Awakening, I immediately wrote it down on a piece of paper.

Discovery/Theory card
The Neutron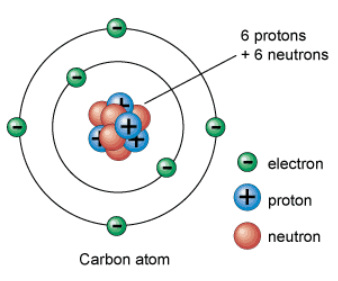
Parker POINTS
A beam of high penetrating power is produced by bombarding beryllium with alpha particles / The beam’s particles have the same mass as protons and are not affected by electric or magnetic fields / Called Neutrons because they have no charge, and are neutral / They are inside and play an important role in maintaining the nucleus.

James Chadwick
Physicist 1891-1947
Parker POINTS
Home: United Kingdom
Awards:Nobel Prize, Copley Medal, & more
Notable Advisor: Ernest Rutherford
Quote:…the statistics and spins of the lighter elements can only be given a consistent description if we assume the neutron is an elementary particle.

Discovery/Theory card
Radioactivity - Polonium and Radium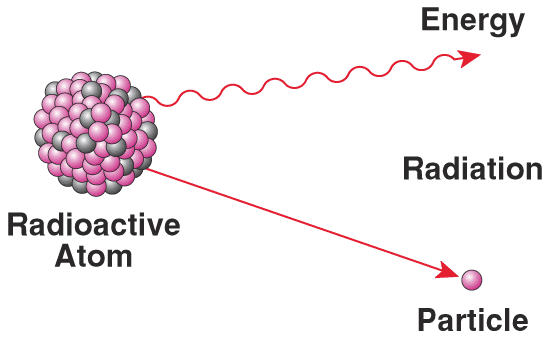
Parker POINTS
The discovery of Polonium (named after Poland) which is 300 times more radioactive than uranium and the discovery of Radium which is several million times more radioactive than uranium / Created the word Radioactivity – which is produced by radioactive elements / They give off heat without a chemical reaction taking place

Robert A. Millikan
1868–1953
Fontaine Chu, Krystal Xie and Ryan Yu POINTS
was an American physicist
he was able to measure the charge on the electron