
Atomic Theory
Chemistry Mix n Match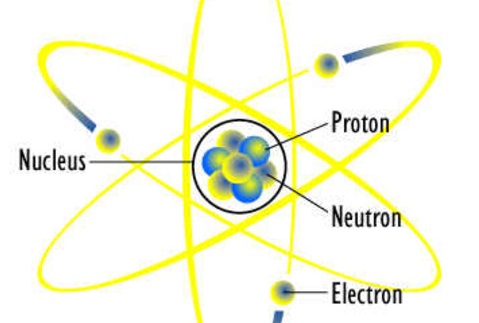
1) All matter is composed of extremely small particles called atoms that can’t be destroyed or created
2)Atom of any element are identical to one another
3)Atoms of different elements combine in specific ratios to form compounds
4)Chemical reactions,atoms are separated,rearranged,and recombined to form new compounds

Neutron
Chemistry Mix n Match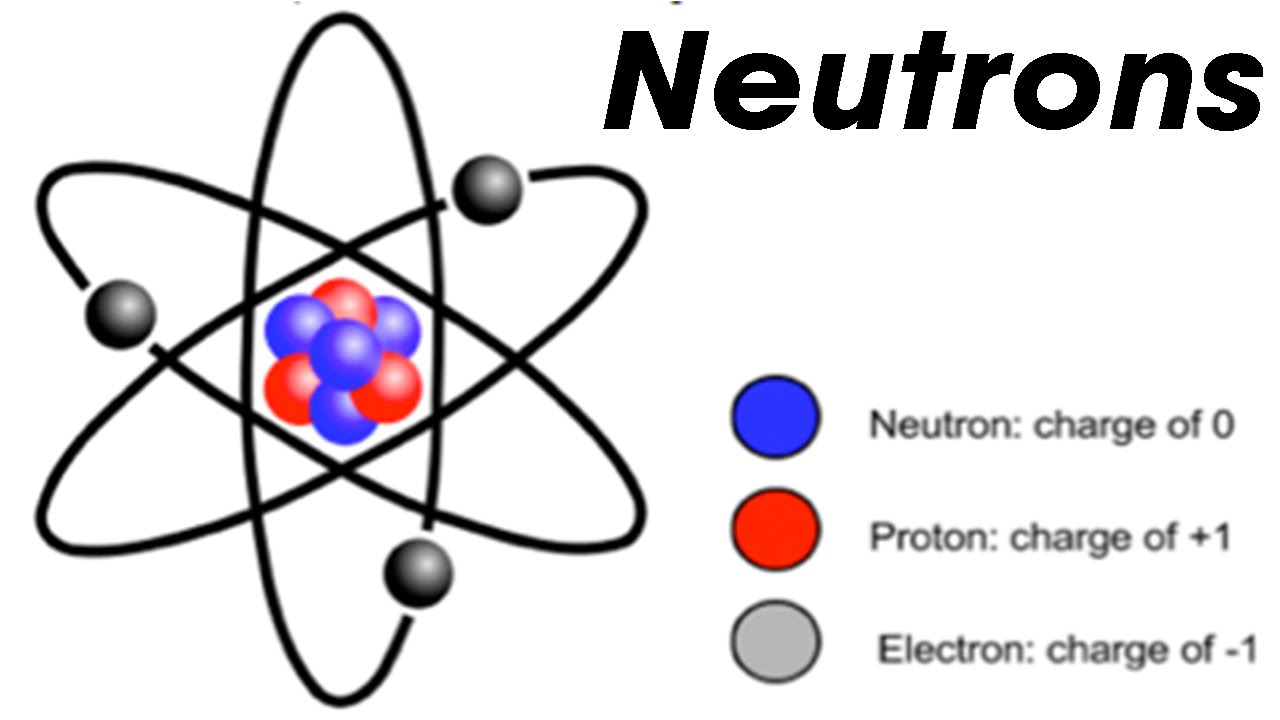
A particle discovered by the Curie-Joliot experiment and first mistaken-ed as gamma rays. It wasn’t effected by magnetic or electric fields and named neutrons because it didn’t posses a charge. It’s found in the nucleus.

Max Planck
Chemistry Mix n Match
A German Physicist in the 1900, proposed that energy could behave like particles in the form of tiny discrete energy packets or bundles he called quanta.

James Chadwick
Chemistry Mix n Match
Demonstrated that the beam Irene Joliot Curie discovered wasn’t gamma rays but was a particle with the same mass as a proton (neutrons).

Robert Millikan
Chemistry Mix n Match
Robert Millikan was the American physicist who discovered the charge of an electron in his “Oil Drop Experiment”.

JJ Thomson
Chemistry Mix n Match
Joseph John Thomson was an English physicist who invented the CRT (Cathode Ray Tube) and identified electrons and the subatomic particle.

Ernest Rutherford
Chemistry Mix n Match
Studied at McGill University in Montreal
Discovered radioactivity involved emission of 2 kinds of particles, alpha and beta.
Discovered that an atom contained a small highly dense positively charged center, known as the nucleus.

Oil Drop Experiment
Chemistry Mix n Match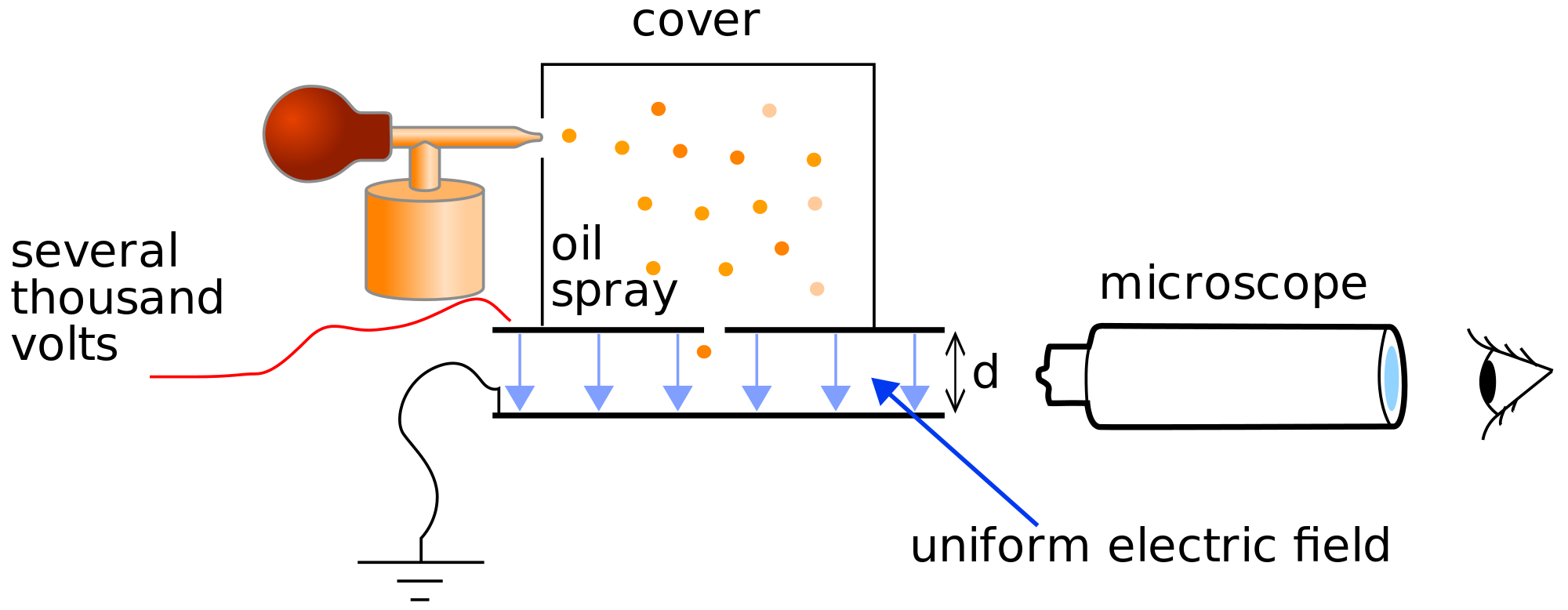
The oil drop experiment was performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the elementary electric charge (the charge of the electron). The experiment entailed observing tiny charged droplets of oil between two horizontal metal electrodes.

Gold foil experiment
Chemistry Mix n Match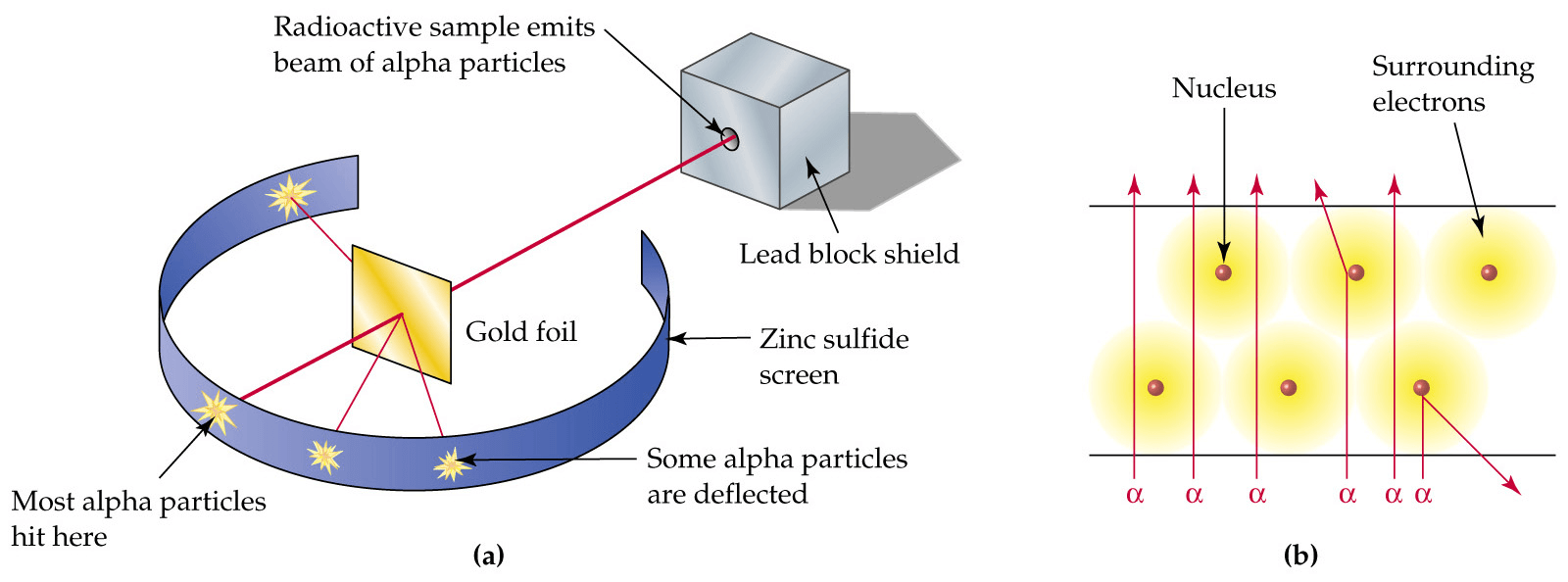
The Rutherford-Geiger Experiment is also known as the Gold Foil Experiment. They were a landmark series of experiments by which scientists discovered that every atom contains a nucleus where its positive charge and most of its mass are concentrated.