
James Chadwick
Physicist 1891-1947
Parker POINTS
Home: United Kingdom
Awards:Nobel Prize, Copley Medal, & more
Notable Advisor: Ernest Rutherford
Quote:…the statistics and spins of the lighter elements can only be given a consistent description if we assume the neutron is an elementary particle.

Discovery/Theory card
Radioactivity - Polonium and Radium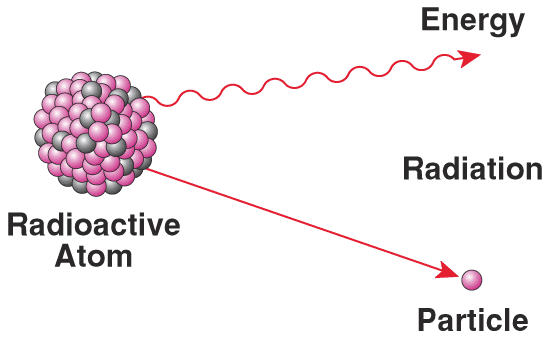
Parker POINTS
The discovery of Polonium (named after Poland) which is 300 times more radioactive than uranium and the discovery of Radium which is several million times more radioactive than uranium / Created the word Radioactivity – which is produced by radioactive elements / They give off heat without a chemical reaction taking place

Robert A. Millikan
1868–1953
Fontaine Chu, Krystal Xie and Ryan Yu POINTS
was an American physicist
he was able to measure the charge on the electron

Marie & Pierre Curie
Physicists 1867-1934 and 1859-1906
Parker POINTS
Home: Poland/France
Awards: Marie: 2 Nobel Prizes (1 shared with Pierre & Henri Becquerel)
Notable Students: Irene Joliot-Curie (daughter)
Quote: Be less curious about people and more curious about ideas

Discovery/Theory card
Artificial Radioactivity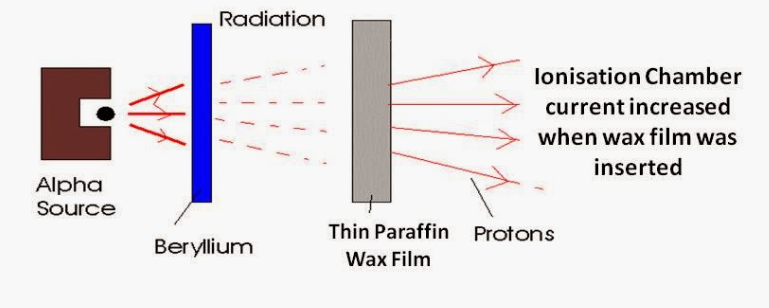
Parker POINTS
Misinterpreting results lead to not discovering the neutron / Starting a new investigation by bombarding aluminum with helium alpha particles converted stable aluminum atoms into radioactive atoms – artificially creating radioactive atoms / Also produced radioactive nitrogen from boron and radioactive silicon from magnesium

Irène & Frédéric Joliot-Curie
Physicists 1897-1956 and 1900-1958
Parker POINTS
Home: France
Awards: Nobel Prize in Chemistry
Notable Advisors: Marie and Pierre Curie (parents)
Quote: For my part, I consider science to be the paramount interest of my life.

Discovery/Theory card
Planetary model of the atom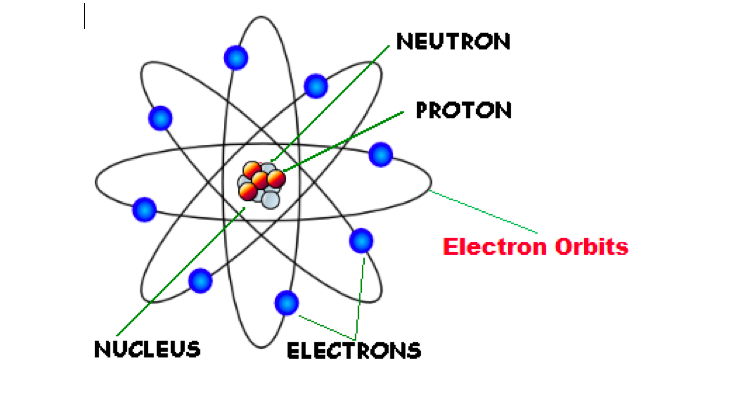
Parker POINTS
Investigating the deflection of alpha particles by atoms lead to rethinking the nature of the atom / Large-angle deflections could not be explained by the old model / The change in the path of some alpha particles is due to a small, densely packed centre of positive charge called the nucleus.

Ernest Rutherford
Physicist 1871-1937
Parker POINTS
Home: New Zealand/United Kingdom
Awards: Nobel Prize in Chemistry, Copley Medal, more
Notable Advisors: JJ Thomson
Quote: All science is either physics or stamp collecting

Discovery/Theory card
The Charge of an Electron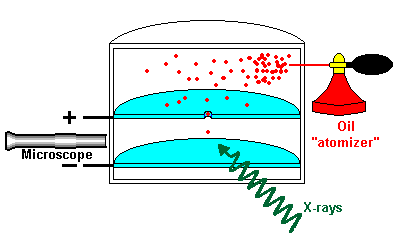
Parker POINTS
Measuring the charge of an electron by spraying oil drops in a chamber and exposing them to X rays, thereby making them positively or negatively charged. Negatively charged oil drops were moved upward by adjusting the electric charge and knowing the weight of the drop, the mass of the electron was discovered.