
Observation of Cathode Ray
Emma, Rachel, Catherine, Mabel POINTS
They observed that in gases at low pressure with an electric charge, the cathode emitted rays. This led to the discovery of the electron.

Work With Cathode Ray Tubes

Emma, Rachel, Catherine, Mabel POINTS
They used this device to study the effect of electricity on gases. Gases are contained at a low pressure, and there is a negative cathode and positive anode. They developed a version of this named after themselves.

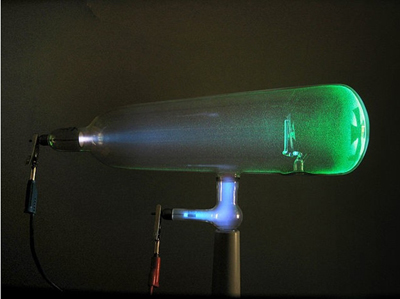
Discovered Thallium

Emma, Rachel, Catherine, Mabel POINTS
They observed an element with an unknown bright green emission line in its’ spectrum, and realized it was a new element. They called this element Thallium, meaning ‘green twig’.

Do You Know Your Scientist?
Scientist Card
Emma, Rachel, Catherine, Mabel POINTS
Sir William Crookes was a Victorian scientist who’s work was fundamental in furthering the development of the atomic theory. Although his main achievements were in Chemistry, he also excelled at physics and astronomy. His deep belief in Spiritualism has cast some of his scientific findings into doubt.

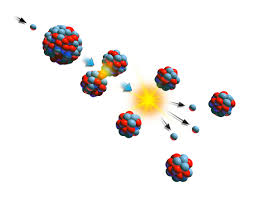
Nuclear Fission
Emma, Rachel, Catherine, Mabel POINTS
Led a team that researched the effect of neutrons on heavy atoms. This led to the discovery of nuclear fission.

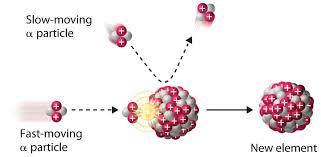
Artificial Radioactive Elements
Emma, Rachel, Catherine, Mabel POINTS
By bombarding the atoms with alpha particles, the successfully created these artificial radioactive elements: radioactive nitrogen from boron, radioactive isotopes of phosphorus from aluminum, and silicon from magnesium.

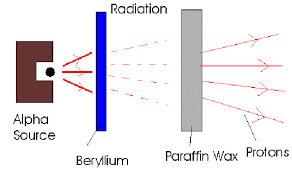
Parafin Wax Experiment
Emma, Rachel, Catherine, Mabel POINTS
By bombarding Beryllium with alpha particles, a high energy beam was emitted that hit a block of paraffin wax and caused it to emit protons. They believed the beam was gamma rays, but Chadwick later proved this beam to be neutrons.

Application of Research
Emma, Rachel, Catherine, Mabel POINTS
Their research in radioactivity had positive implications in the medical industry, as radioactive materials were being used more often. Being able to create radioactive materials artificially helped lower the cost.

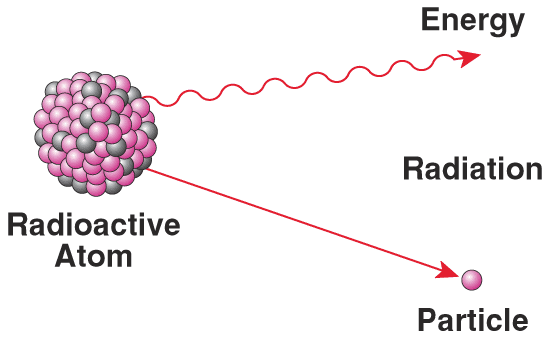
Creation of Radioactive Isotopes
Emma, Rachel, Catherine, Mabel POINTS
By bombarding light elements such as Beryllium with alpha particles, artificial radioactive isotopes were formed from stable elements.