DIY Cards

Discovery/Theory card
The Plum Pudding Model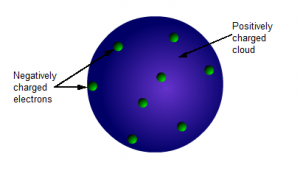
Parker POINTS
The plum pudding model was first proposed in 1904.
It is a positively charged ball that has negative electrons evenly spread throughout it.
It is an important step in atomic theory because it is the first model that included the electron and indicated that atoms were made of smaller units.

James Chadwick ( Mina )
Julia, Vivian, Winnie, Mina
3 POINTS
– The British Scientist who discovered the neutron in 1932 and won the Nobel Prize in 1935
– Was the leader of the British team in the Manhattan Project to create the world’s first nuclear bomb

Laid Foundation for Thomson

Emma, Rachel, Catherine, Mabel POINTS
This scientists’ work with cathode rays prompted Thomson to observe that the ray was negatively charged, and subsequently discover the electron.

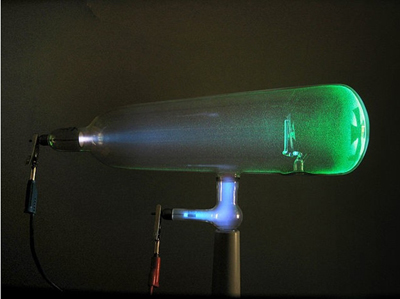
Observation of Cathode Ray
Emma, Rachel, Catherine, Mabel POINTS
They observed that in gases at low pressure with an electric charge, the cathode emitted rays. This led to the discovery of the electron.

Work With Cathode Ray Tubes

Emma, Rachel, Catherine, Mabel POINTS
They used this device to study the effect of electricity on gases. Gases are contained at a low pressure, and there is a negative cathode and positive anode. They developed a version of this named after themselves.

Discovered Thallium

Emma, Rachel, Catherine, Mabel POINTS
They observed an element with an unknown bright green emission line in its’ spectrum, and realized it was a new element. They called this element Thallium, meaning ‘green twig’.