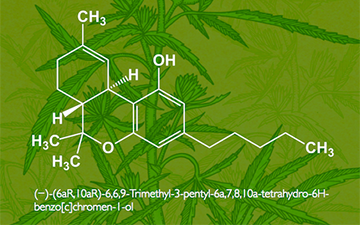
Tetrahydrocannabinol (THC), or more precisely its main isomer (−)-trans-Δ9-tetrahydrocannabinol ( (6aR,10aR)-delta-9-tetrahydrocannabinol), is the principal psychoactive constituent (or cannabinoid) of cannabis. First isolated in 1964 by Israeli scientists Prof. Raphael Mechoulam and Dr. Yechiel Gaoni at the Weizmann Institute of Science[8][9][10] it is a water-clear glassy solid when cold, which becomes viscous and sticky if warmed. A pharmaceutical formulation of (−)-trans-Δ9-tetrahydrocannabinol, known by its INN dronabinol, is available by prescription in the U.S. and Canada under the brand nameMarinol. An aromatic terpenoid, THC has a very low solubility in water, but good solubility in most organic solvents, specifically lipids and alcohols.[6] THC, CBD,CBN, CBC, CBG and about 80 other molecules make up the phytocannabinoidfamily.
Like most pharmacologically-active secondary metabolites of plants, THC inCannabis is assumed to be involved in self-defense, perhaps againstherbivores.[11] THC also possesses high UV-B (280–315 nm) absorption properties, which, it has been speculated, could protect the plant from harmful UV radiation exposure.[12][13][14]
Tetrahydrocannabinol, along with its double bond isomers and their stereoisomers, is one of only three cannabinoids scheduled by the Convention on Psychotropic Substances (the other two are dimethylheptylpyran andparahexyl). Cannabis as a plant is scheduled by the Single Convention on Narcotic Drugs (Schedule I and IV).
(From Wikipedia, June 2015)