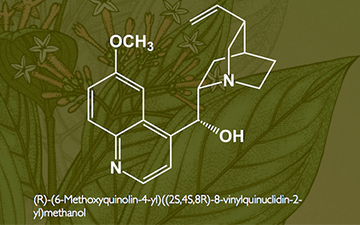
Quinine (US /ˈkwaɪnaɪn/, /kwɪˈniːn/ or UK /ˈkwɪniːn/ kwin-een) is a white crystalline alkaloid having antipyretic (fever-reducing), antimalarial, analgesic (painkilling), and anti-inflammatoryproperties and a bitter taste. It is a stereoisomer of quinidine, which, unlike quinine, is an antiarrhythmic. Quinine contains two major fused-ring systems: the aromatic quinoline and thebicyclic quinuclidine.
Quinine occurs naturally in the bark of the cinchona tree, though it has also been synthesized in the laboratory. The medicinal properties of the cinchona tree were originally discovered by the Quechua, who are indigenous to Peru and Bolivia; later, the Jesuits were the first to bring cinchona to Europe.
Quinine was the first effective Western treatment for malaria caused by Plasmodium falciparum, appearing in therapeutics in the 17th century. It remained the antimalarial drug of choice until the 1940s, when other drugs such as chloroquine that have fewer unpleasant side effects replaced it. Since then, many effective antimalarials have been introduced, although quinine is still used to treat the disease in certain critical circumstances, such as severe malaria, and in impoverished regions due to its low cost. Quinine is available with a prescription in the United States and “over-the-counter” (in minute quantities) in tonic water. Quinine is also used to treat lupus and arthritis. Quinine was also frequently prescribed in the US as anoff-label treatment for nocturnal leg cramps, but this has become less prevalent due to a Food and Drug Administration statement warning against the practice.[2]
Quinine is highly fluorescent (quantum yield ~0.58) in 0.1 M sulfuric acid solution and it is widely used as a standard for fluorescence quantum yield measurement.[3][4] It is on the WHO Model List of Essential Medicines, a list of the most important medications needed in a basic health system.[5]
(From Wikipedia, July 2015)